Fee Schedule
About PAAB
The Pharmaceutical Advertising Advisory Board (PAAB) is an independent not-for-profit organization funded on a fee-for-service model. It is the only regulator whose preclearance service is recognized by Health Canada for advertising directed to healthcare professionals. PAAB works to protect Canadians by ensuring that healthcare product advertising meets the regulatory, scientific, therapeutic, and ethical standards outlined in the Code of Advertising Acceptance. All PAAB approved materials bear the PAAB logo.
Base fees for PAAB reviews
The fee calculator on the PAAB website can also help you anticipate how any relevant combination of submission properties / attributes can impact the review cost.

Messenger is now available for all submissions! Additional fees apply.
a) Preclearance reviews based on the PAAB Code (directed to HCP/Patient)
Current Fees
Base fee for ALL APS
EXCEPT for "Series" & "Minor update" APS
English or French
English and French
English or French
English and French
Standard
$443
$512
$159
$216
ARO-10
$545
$614
$182
$239
ARO-7
$665
$766
$216
$272
ARO-4
$886
$1,022
$330
$386
ARO-2
$1,329
$1,534
$443
$512
Please either speak with a PAAB file coordinator or send an email to review@paab.ca if considering ARO-2 for an APS that does not meet BOTH of the following criteria (to ensure that we can deliver the first response within 2 business days):
- 10 or less pages of new content
- 5 or fewer references requiring detailed assessment
As a general guide, a “detailed assessment” entails needing to read the entire reference to determine its validity (e.g., clinical trials, surveys) and/or to ensure that the promoted elements are not overly selective. Factors that typically determine whether a detailed assessment will be required include whether the reference has been used for similar claims in prior PAAB approved materials, and the nature of the reference (e.g., new studies & surveys generally require a detailed assessment). Additional information will be provided in the upcoming version of the Submission Guide.
Pre-NOC Submissions - Additional Fees
See
Administrative Guideline for the Review of Pre-NOC Advertising Submissions for complete details on pre-NOC submissions.
| Update Type | Turnaround Time | Additional Fee* |
|---|---|---|
| The PM or unsolicited APS update does not impact the APS (e.g. grammatical changes, the drug receives a name, measurement units change, etc.) | Continues in the same eFile 3-day standard revision turnaround time | Not applicable |
| The PM or unsolicited APS update has a minor impact on the APS (e.g. bullet added to fair balance, adverse event (AE) is added to an existing AE table, etc.) | Continues in the same eFile 3-day standard revision turnaround time | +$200.00 (2 languages) or +$147.00 (single language) per round of revision with a PM update or unsolicited change that has a minor impact on the APS |
| The PM or unsolicited APS update has a significant impact on the APS (e.g. indication change, new data, new creative concept, etc.) | Requires resubmission as a new eFile 10-day turnaround time (instead of 15 day pre-NOC initial turnaround time) to facilitate continuation of the eFile | +full standard fee for a PM update or unsolicited change with a significant impact on the APS |
*All fees are subject to change.
b) Direct to Consumer Advertising or Information (DTCA/I) reviews
Current Fees
Base fee for ALL APS
EXCEPT for "Series" & "Minor update" APS
English or French
English and French
English or French
English and French
Standard (4-day)
$443
$512
$159
$216
ARO-4
$545
$614
$182
$239
ARO-2
$730
$850
$216
$272
See the guidance document on Risk Management Tools
c) Assessment of Risk Management Tools (HCP/Patient)
Current Fees
Base fee for ALL APS
EXCEPT for "Series" & "Minor update" APS
English or French
English and French
English or French
English and French
Standard (4-day)
$443
$512
$159
$216
ARO-4
$512
$579
$182
$239
ARO-2
$665
$766
$216
$272
See the guidance document on Risk Management Tools
d) Request for Written Opinion
Current Fees
Standard (4-day)
$443
e.g., assessment of creative, assessment of a single clinical trial and corresponding claims, assessment of a novel approach/media/platform, determination of whether a piece is advertising or information, and so on
See the following relevant advisories:
Starting in 2023, all PAAB fees will be adjusted annually by the prior year’s change in cost of living. These adjustments will impact all files submitted as of the first working day of each year. All fees are exclusive of HST.
Fees are invoiced after the first review letter has been sent. Fees are for the cost of the review and not for the acceptance of the APS
See the PAAB advisory “Increased specificity in series fee criteria” for APS that are eligible for the series fee.
Supplementary fees for PAAB reviews
Current Fees
Standard
ARO-10
ARO-7
ARO-4
ARO-2
Supplemental Length/Reference Fee
APS is more than 10 pages or more than 15 references
$230
+ $2 per "new content page"
$230
+ $8 per "new content page"
$230
+ $4 per "new content page"
Applicable to Annex 1a-d EXCEPT:
Straight renewals or Minor updates
Resubmissions to approval
Invoiced upon receipt of the third resubmission
$156 per APS requiring three or more resubmissions:
The resubmission count will exclude one layout assessment and one translation assessment.
Additional fees
Iterations - 1 iteration is included with the standard submission fee
English
or
French: $159
English and French: $216
Modular Submissions - does not include the modular library
English
or
French: $159
English and French: $216
messaging (Decoupled assessments) ¥
≤ X client messages per resubmission per APS
N/A
> X client messages per resubmission per APS
N/A
$125
¥ Feature is only available on AROs
Starting in 2023, all PAAB fees will be adjusted annually by the prior year’s change in cost of living. These adjustments will impact all files submitted as of the first working day of each year. All fees are exclusive of HST
PAAB meeting and training fees
Current Fees
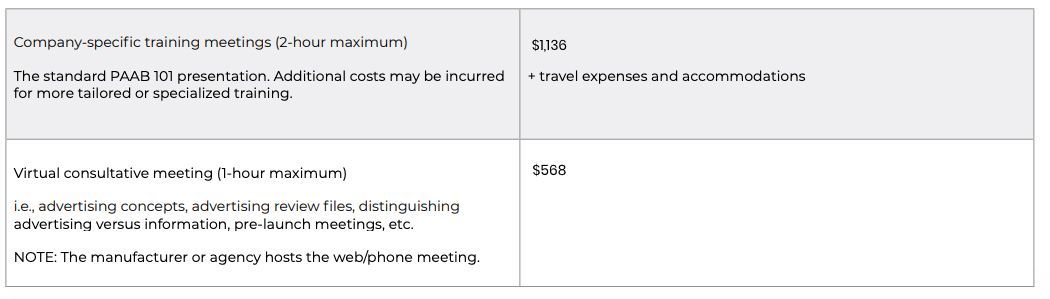
Starting in 2023, all PAAB fees will be adjusted annually by the prior year’s change in cost of living. All fees are exclusive of HST.
Fees are invoiced after the first review letter has been sent. Fees are for the cost of the review and not for the acceptance of the APS. Once a piece has an approval number, we consider the approval process to have been completed for the fee that was assessed. Any revisions after that will be treated as a new submission with a new file number and billed a full fee. A review of prescribing information at launch or when revised will be billed an "All APS" fee.
All APS review files which have not received any response for over 180-days will be closed and any revisions following the 180-day period will be assigned a new file number and subject to a new fee.
All APS review files that are not completed within a period of twelve months will be assigned a new file number and subject to a new fee.
Once an acceptance number is issued, the file is considered completed and further revisions to the APS would require a new file number and subject to a new fee.
Invoices are payable within 30 days; advertisers with outstanding balances may be required to clear their accounts before new reviews can begin. Our BN is 104174743.
Questions about fees should be directed to the PAAB office: 203-1305 Pickering Parkway, ON L1V 3P2.
Tel: (905) 509-2275
Send email to review@paab.ca.
Advisory: Increased specificity in series fee criteria

