| Item No | Checklist Item (clients can use this tool to help make decisions regarding use of Pharmacoeconomic studies in advertising claims) | ✓ |
| What Must Appear in the Advertisement or Promotional System | ||
| 3.1 | Consistency with Terms of Market Authorization | |
| 3.6 | Describe the comparator | |
| 3.7 | Report the discount rate applied | |
| 3.11 | Describe costs and outcomes of interest separately | |
| 3.12 | Qualify claims of cost-effectiveness when the therapy is associated with increased costs |
|
| 3.14 | Describe the source of funding | |
| Must Appear in the Published Study for Claim Validation | ||
| 3.1 | Consistency with Terms of Market Authorization | |
| 3.2 | Describe the study objective in the form of an answerable question | |
| 3.3 | State and justify the form of the analysis | |
| 3.4 | Describe the perspective (e.g. health system, payer, society) and how this relates to the decision |
|
| 3.5 | Describe the source for estimating measures of effectiveness | |
| 3.6 | Describe the comparator | |
| 3.7 | Describe the methods for measuring and valuing preference-based outcomes | |
| 3.8 | Report the discount rate applied | |
| 3.9 | Describe the approach to measuring resource use and costs | |
| 3.10 | Describe mean values for main categories of costs and outcomes of interest |
|
| 3.11 | Describe costs and outcomes of interest separately | |
| 3.12 | Qualify claims of cost-effectiveness when the therapy is associated with increased costs | |
| 3.13 | Describe the effect of uncertainty on the study findings | |
| 3.14 | Describe the source of funding | |
1. Key Benefits:
Economic claims provide additional information to aid HCPs in making appropriate therapeutic choices that may not be captured in claims based solely on clinical evidence. They aim to provide decision makers with one or more measures of value, compared with one or more measures of resource use, so that decision makers can decide whether or where resources are best spent.
2. Key Pitfalls:
Claims based on economic evaluation are susceptible to error that may not be easily detected without adequate disclosure of the structure and underlying parameters useful to their interpretation.
3. Managing pitfalls:
The following checklist can be used to guide industry and the PAAB staff in determining whether economic findings may appear within advertising/promotional systems (APS). The checklist relates only to factors specific to the reporting of economic studies that attempt to make comparisons (i.e. economic evaluations). It is assumed that economic claims are consistent with studies conducted according to National Standards (see cadth.ca/media/pdf/186_EconomicGuidelines_e.pdf). It is also assumed these studies will be based on study designs that are deemed acceptable to the PAAB, such as randomized controlled trials and not be based on designs, such as mathematical modeling studies, that are not considered acceptable. Refer to the PAAB code for general factors relating to acceptability of a study.
❐ 3.1 Consistency with Terms of Market Authorization
Principles:
Drug advertising should be consistent with the Health Canada approved Terms of Market Authorization (TMA).
Rationale:
Advertising content which is inconsistent with the TMA, would contravene section 9.1 of the Food and Drugs Act.
Application:
Economic evaluations cannot be used to support observations that contradict anything in the TMA (with respect to magnitude. direction, or duration of clinical effect).
❐ 3.2 Describe the study objective in the form of an answerable question
Principle:
Economic evaluations are intended to support decision-making. Their objectives should translate into a question in answerable form that is relevant to the decision problem of competing therapeutic strategies.
Rationale:
It is possible to use economic information to demonstrate a comparative claim for which the study was never intended to measure.
Application:
Describe the competing strategies being compared with details of the specific interventions being compared (drug, form, dose etc.), the health care setting and the target (patient) population. It is not enough to state that ‘The purpose of the study was to assess the cost-effectiveness of treatment X’. Examples of acceptable and unacceptable questions are available in the Appendix to this Supplement.
❐ 3.3 State and justify the form of the analysis
Principle:
In order to be useful for consistent decision making, economic evaluations should be consistently conducted.
Rationale:
National standards for the conduct of economic evaluation state cost-utility analyses must be conducted where meaningful differences in health-related quality of life (HRQL) between the intervention and comparators have been demonstrated. Otherwise, effectiveness measures should be important outcomes to the health of patients.
Application:
State whether the analysis is a: cost-effectiveness, cost-utility, cost-consequences, cost-benefit, or cost-minimization analysis. Justify why a cost-utility analysis was not conducted by declaring no knowledge of differences in HRQL across treatment groups.
❐ 3.4 Describe the perspective (e.g. health system, payer, society) and how this relates to the decision
Principle:
Economic evaluations useful for decision-making should capture costs and consequences relevant to the decision problem.
Rationale:
It is possible to get very different findings from economic evaluations that use different perspectives, and some perspectives may be inappropriate for a given decision problem. Additionally, National Standards suggest results from a third-party payer perspective must always be stated, even if a societal perspective has also been taken.
Application:
Describe the perspective (i.e. health system, payer, society) in terms of costs included, the associated components (i.e. direct medical costs, direct non-medical costs, indirect/productivity costs), and how this relates to the decision problem. At minimum, report findings from a third party payer perspective that includes the following cost components: 1) direct costs to the publicly funded health care system; 2) direct costs to patients and families; 3) time costs to patients and families
❐ 3.5 Describe the source for estimating measures of effectiveness
Principle:
Measures of effectiveness must be derived from studies that are allowable in advertising.
Rationale:
The design and conduct of a single study leads to the potential for the introduction of error (random, systematic and measurement), as well as the potential for error from confounding. As with any comparative effectiveness claim, adequate description of the study underlying the economic claim must be made available to allow proper scrutiny.
Application:
Refer to the appropriate sections of the PAAB Code for guidance on what information must be available for any given study design. This also applies to estimates from study subgroups. These could include availability of study protocols, methods of selection of study population, methods of allocation of study subjects, whether intention to treat analysis was used, recruitment period, missing data methods, time of follow-up, and methods for handling potential biases in the study design (i.e. selection biases). It is important to justify why the single study was selected as a source of clinical effectiveness data versus other available sources of information.
❐ 3.6 Describe the comparator
Principle:
Not knowing what the treatment is being compared to is unhelpful for decision making
Rationale:
The cost-effectiveness of any intervention varies according to the costs and consequences of what it is being compared to. The price of the comparator is a particularly important feature in economic evaluations of drug-based therapeutic strategies.
Application:
State either 1) the brand name or 2) the generic name and manufacturer of the comparator drug used for deriving cost information. If the economic evaluation is based on a comparison with placebo, state “placebo”. For generic-branded items with multiple manufacturers available at a similar price, it is appropriate to state “generic brand” instead of describing an individual manufacturer.
❐ 3.7 Describe the methods for measuring and valuing preference-based outcomes
Principle:
Preference-based measures are useful for conducting economic evaluations that provide effectiveness measures that are transferable across therapeutic specialties. They provide a more interpretable metric of value for money.
Rationale:
Different methods used to measure and value preferences can lead to different economic findings.
Application:
Describe the method of measurement of preference-based outcomes, for example, the use of generic instruments (i.e. EQ-5D, SF-6D), a direct scaling technique (i.e. standard gamble approach, time trade-off approach), contingent valuation, discrete choice experiment, etc. The format and timing(s) of these measurements should also be described. The source of preferences (i.e. general population) for valuation should also be described. To avoid double-counting, subjects in exercises measuring preferences should be asked to value lost leisure time in terms of changes in preferences, and to assume that health care costs and income losses are fully reimbursed. A representative sample of the general public, suitably informed, is the preferred source for preferences. Measures based on willingness to pay may only be presented in addition to these.
❐ 3.8 Report the discount rate applied
Principle:
Societies have preferences for health effects and resource consumption that vary with time.
Rationale:
Different discount rates can lead to different economic findings. National standards for economic evaluation have clear guidance on discounting in economic evaluation.
Application:
Analysts should apply a 5% discount rate to calculate present values of future costs and/or health outcomes beyond one year. A 0% and 3% should be used for sensitivity analysis.
❐ 3.9 Describe the approach to measuring resource use and costs
Principle:
Costs, compared with other relevant consequences of an intervention, are the central focus of economic evaluation.
Rationale:
Different methods for estimating and valuing costs can lead to different economic findings. National standards for economic evaluation have clear guidance on measuring and valuing costs related to resource use in economic evaluation.
Application:
Describe approaches to estimating resource use, for example, trial data collection forms, separately designed questionnaires, data extracted from routine data collection systems, etc. Indicate settings for collection outside of Canada. Report dates for the estimation of resource quantities and unit costs. Identify the currency and year of currency in which costs are reported. Describe methods for adjusting prices (i.e. general or medical consumer price index). Describe methods for converting costs into a common currency base (i.e. purchasing power parities) and the exchange rate. Transfer payments should be excluded from the public payer and societal perspectives. Protocol-driven costs taken from clinical trials and unrelated costs that are incurred during normal life-years should be excluded from the evaluation.
❐ 3.10 Describe mean values for main categories of costs and outcomes of interest
Principle:
Aggregated costs are insufficient for determining the validity of a claim, which may be entirely driven by a single cost category associated with high levels of variability or of limited relevance.
Rationale:
The costs and consequences associated with clinical decisions must be examinable, to determine the credibility of a claim.
Application:
Report mean values for the main categories of costs and outcomes of interest, as well as mean differences between the comparator groups.
❐ 3.11 Describe incremental costs and outcomes of interest separately
Principle:
Incremental cost-effectiveness ratios or net benefits are insufficient for decision-making.
Rationale:
The incremental costs and consequences contributing to an incremental cost-effectiveness ratio or net benefit measure must be reported as their magnitude is relevant for decision-making.
Application:
Report mean values of total costs and outcomes of each strategy separately along with the incremental costs and outcomes. If the costs and outcome do not apply to an average individual patient, report the number of patients used to calculate these. For more than two mutually exclusive strategies, incremental costs and consequences must be derived from 1) eliminating strategies that are more costly and less effective than the least costly and effective strategy and 2) comparing the strategy to the next most effective and costly program. Incremental costs and outcomes must be apparent to the reader. It is acceptable to have mean values of costs and outcomes appear in a footnote.
❐ 3.12 Qualify claims of cost-effectiveness when the therapy is associated with increased costs
Principle:
The term “cost-effective” has multiple meanings and must be qualified.
Rationale:
The term “cost-effective” is allowed for comparisons that satisfy one of two specifications: 1) therapeutic strategies that are associated with equal or increased measures of clinical effectiveness along with reductions in costs; OR 2) therapeutic strategies that are associated with increased measures of clinical effectiveness along with increases in costs that are less than a specific measure of opportunity cost for the health system with an exogenous budget constraint, such as the cost-effectiveness of a marginal health care program that will be displaced. A common measure of opportunity cost is the cost-effectiveness threshold, which is the reciprocal of the shadow price of a single budget constraint or multiple budgets over time.
Application:
When a therapy is reported to be “cost-effective” and is associated with increased costs, this must be accompanied by a similarly-sized declaration of the measure of opportunity cost used to qualify the claim. This can be phrased as either a “cost-effectiveness threshold” or a decision-maker’s “willingness to pay” for an extra unit of effectiveness. (See Examples in Appendix)
❐ 3.13 Describe the effect of uncertainty on the study findings
Principle:
It is not possible to understand the robustness of the claim without an appropriate analysis of uncertainty.
Rationale:
The findings of economic evaluation may be sensitive to key parameters relating to resource use and effectiveness. For example, the costs and consequences may be very sensitive to assumptions about hospitalization costs, or drug price, which require scrutiny.
Application:
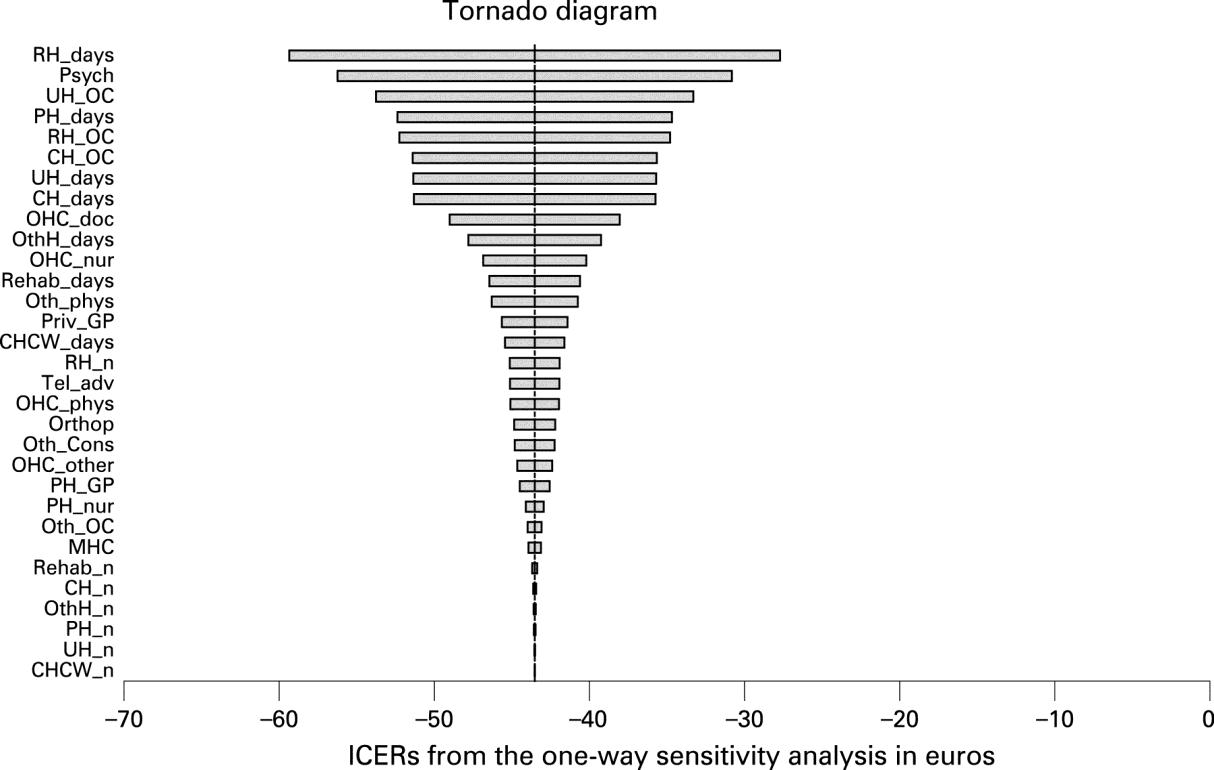
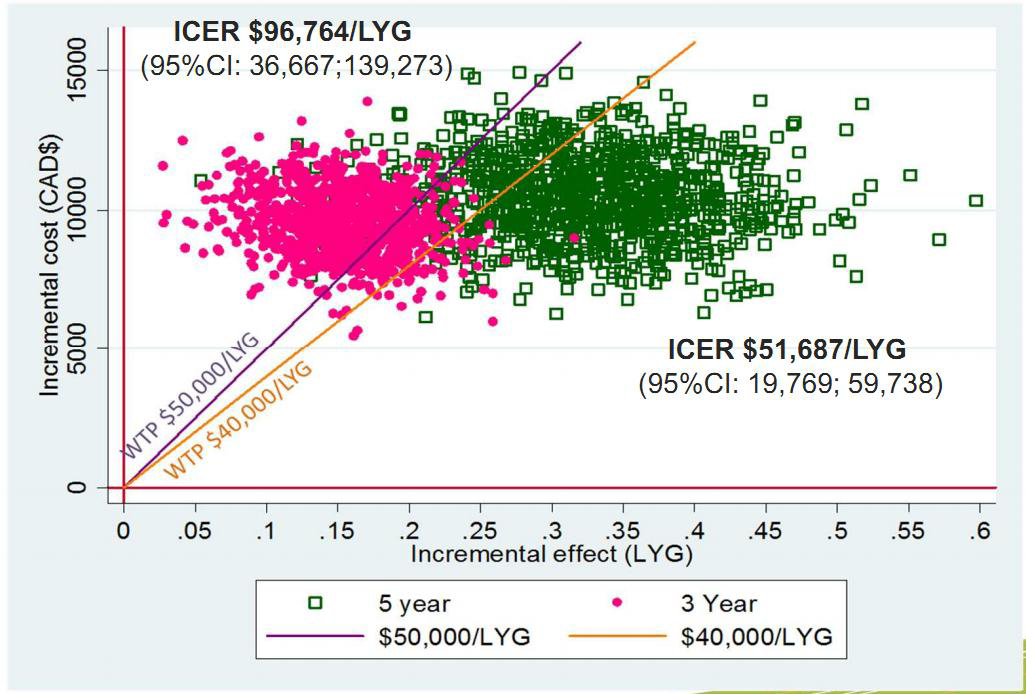
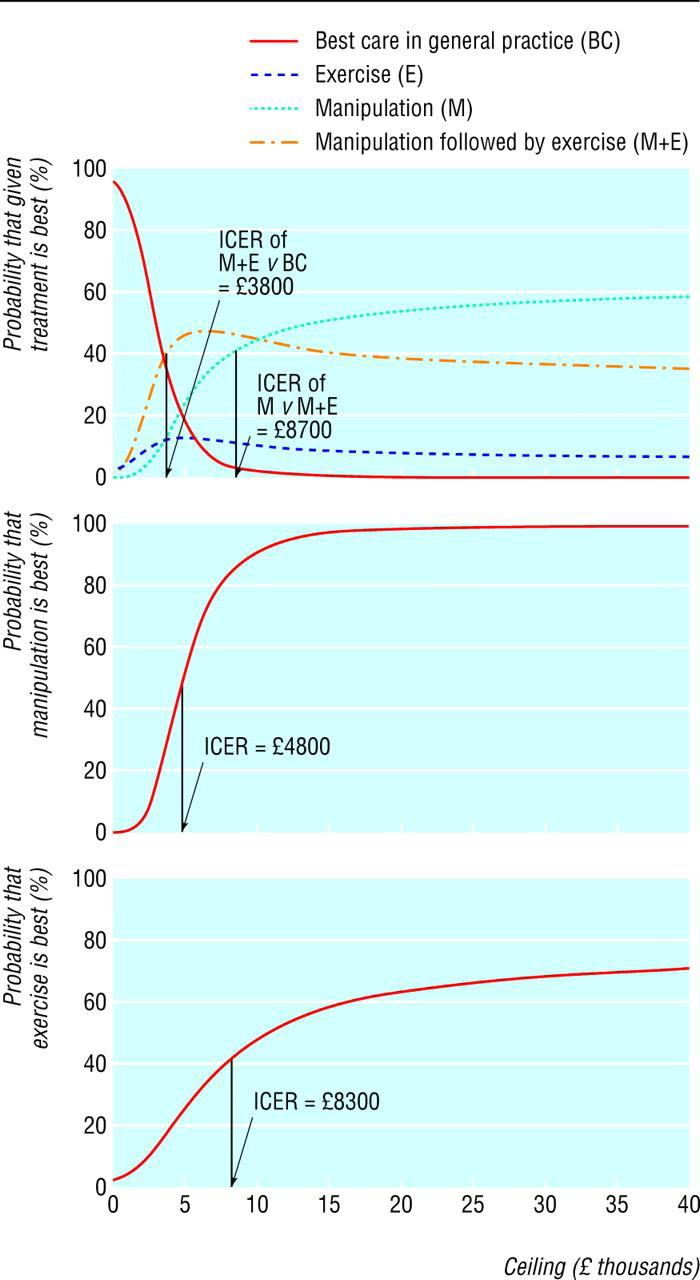
Describe the effects of sampling uncertainty for the estimated incremental cost and incremental effectiveness parameters together with the impact of methodological assumptions (i.e. time horizon, study perspective). Either a univariate (deterministic) or multivariate (probabilistic) sensitivity analysis are appropriate. At minimum, a univariate sensitivity analysis should be presented using a tornado diagram that includes all cost-effectiveness parameters. For probabilistic sensitivity analyses, a scatter plot or cost-effectiveness acceptability curve are appropriate. The values, ranges, references, and if used, distributional forms for all parameters should be provided to allow interpretation. Examples are provided in the Appendix.
❐ 3.14 Describe the source of funding
Principle:
The validity of the claim must be interpreted through an understanding of the role of intervention sponsors.
Rationale:
There is a large body of evidence that shows more exaggerated claims of cost-effectiveness are associated with who has funded the economic evaluation. Claims of cost-effectiveness with a statement of study sponsorship allow decision makers to gauge the accuracy of the information.
Application:
Describe how the study was funded.
Relating to 3.2: Examples for describing the study objective in the form of an answerable question
Relating to 3.11: Examples for qualify claims of cost-effectiveness when the therapy is associated with increased costs
![]()
Never miss an update. Get the latest PAAB info delivered right to your email address.
In an effort to constantly serve our clients better, PAAB has unveiled a new electronic submission process(eFiles). Effective January 2, 2008 all submissions will have to be submitted via the eFiles system. Please have a Senior Official (Director level) send an email to the administration team at review@paab.ca with the contact information of the person(s) who will be designated as administrator(s) for your company. Click on eFiles, on the menu, then eFiles Tutorial for a tutorial on how eFiles works.
Please contact the admin team at PAAB if you need assistance with eFiles
The Accelerated Preclearance Pathway
Learn more and share your feedback by April 14
Click here to provide feedback