UPDATE:
Febraury 1, 2024
The Code update, Guidance on When the Attention Icon is Required and Its Presentation, and Guidance on Real-World Evidence/Data are now in effect. Please ensure that you are referencing back to these links for the finalized versions of the documents.
We look forward to working with you on this updated approach to the sharing of clinical data.
___________________________________________________________________________________________
December 1, 2023
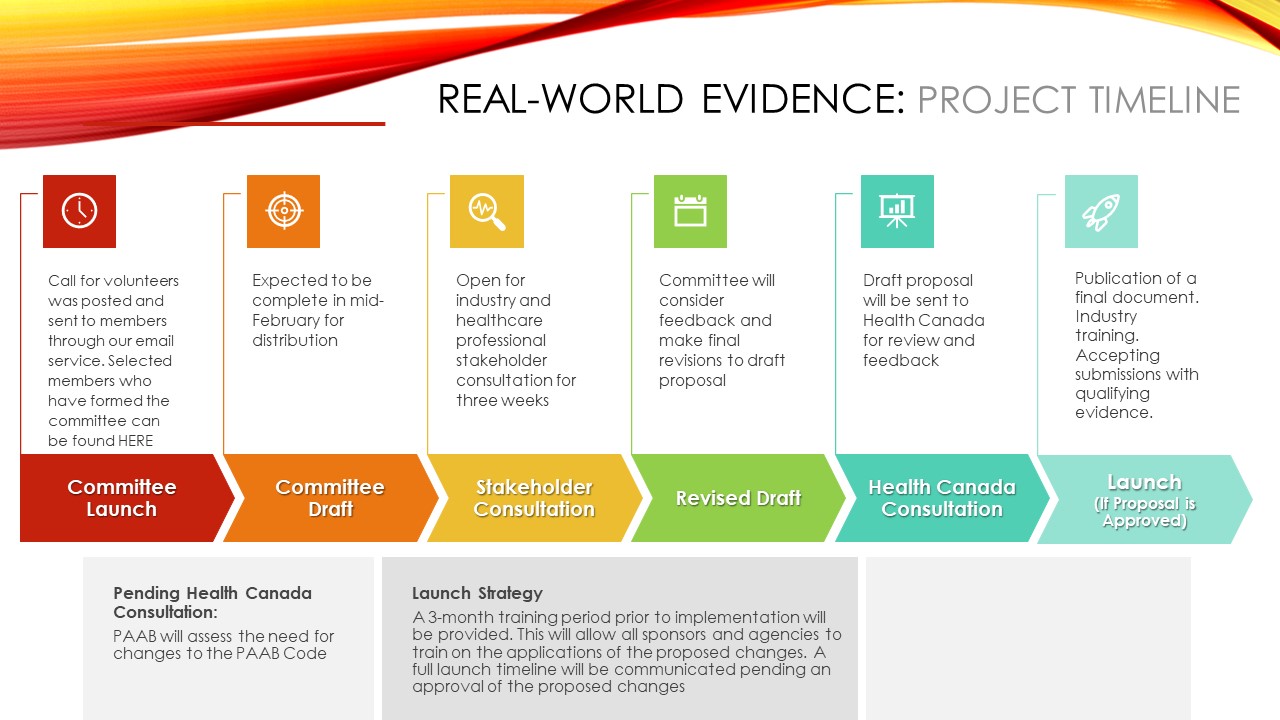
PAAB is excited to announce that the Board has approved the changes to the Code that will allow for the application of the RWE guidance document as well as the use of those same formatting requirements for other data presentations such as subjective endpoints from open-label studies.

The Code change will come into effect on February 1st 2024. PAAB will not be accepting presentations with data under the Code changes until this date, to allow all companies adequate time to train their staff and assess their portfolios.
PAAB will be working over the next month, to create some additional training documents to assist in application of the new guidances and Code updates and removing “draft” watermark. The Code will be updated to reflect the new wording (found here) on February 1st.
We would like to thank the RWE Committee once again for all their work and feedback throughout the process, as well as the manufacturers, agencies, and associations who provided extensive feedback throughout the rounds of revisions. We feel the new framework will allow for the inclusion of RWE in advertising in a truthful, transparent and trustworthy manner that will work in the best interest of all Canadians who rely on informed healthcare professionals. With your collaboration, we believe this places Canada at the forefront of marketing best practices and we could not have done it without you.
We look forward to working with you throughout the implementation. Let us know what you think below.
___________________________________________________________________________________________
October 13, 2023:
PAAB is pleased to announce that the draft PAAB Guidance on Real-World Evidence/Data has been forwarded on to Health Canada for consultation. In addition to the RWE document, PAAB has also leveraged the work of the committee to inform additional proposed adjustments to review practices in order to further expand the information that can be shared with HCPs.
Both draft documents and proposed Code updates can be found here.
___________________________________________________________________________________________
Please remember that this is a draft guidance. It should not be shared externally.
___________________________________________________________________________________________
In November of 2022, PAAB struck a committee to generate a framework for acceptance of Real-World Evidence (RWE) in healthcare professional advertising. While the framework will focus on evidentiary standards and disclosure criteria pertaining to RWE, it will set the stage for guidance pertaining to other forms of evidence which don’t meet evidentiary gold standards but have value to clinical practice. The committee is dedicated to ensuring the framework promotes the use of RWE in advertising in a manner that is truthful, transparent, trustworthy, and in the best interest of all Canadians who rely on informed healthcare professionals.
The committee consists of industry experts from manufacturers and agencies.
|
Amyn Sayani Ph.D. Director, Medical Evidence Scientific Affairs AstraZeneca Canada |
Chrysanthy Christopoulous Medical Advisor and Strategist Lemieux Bedard |
|
|
Jefferson Tea Vice-President, Medical & Scientific Affairs Takeda |
Matt Slipek Sr Planner, Strategic Planning Havas |
|
|
Manushvi Gupta Regulatory Manager Reckitt
|
Nina Hemery Medical Advisor Fisika |
|
|
Virginie Giroux Director of HEOR Merck |
|
|
|
|
|
|
|
PAAB |
Patrick Massad – Commissioner |
|
|
|
Pauline Dong - Director of Policy |
|
|
|
Jennifer Carroll - Director of Communications |
|
As the committee finalizes the draft document, we will head to industry consultation and consultation with healthcare professionals. Please stay tuned for further development and updates along the development timeline.
![]()
Never miss an update. Get the latest PAAB info delivered right to your email address.
In an effort to constantly serve our clients better, PAAB has unveiled a new electronic submission process(eFiles). Effective January 2, 2008 all submissions will have to be submitted via the eFiles system. Please have a Senior Official (Director level) send an email to the administration team at review@paab.ca with the contact information of the person(s) who will be designated as administrator(s) for your company. Click on eFiles, on the menu, then eFiles Tutorial for a tutorial on how eFiles works.
Please contact the admin team at PAAB if you need assistance with eFiles
The Accelerated Preclearance Pathway
Learn more and share your feedback by April 14
Click here to provide feedback